Temperature Unit Converter
Temperature, that intangible quality that determines everything from the weather to chemical reactions in our bodies, has been an object of scientific fascination for millennia. Its study represents one of humanity's most significant intellectual endeavors, one that intertwines physics, philosophy, and technological ingenuity.
Temperature, in its purest form, is a measure of the average kinetic energy of the particles that make up a substance. This microscopic definition is expressed mathematically as:

Where:
-
⟨Ek⟩ = average kinetic energy per particle
-
kB = Boltzmann constant (1.380649 × 10⁻²³ J/K)
-
T = absolute temperature in Kelvin
This fundamental relationship connects the observable world with the quantum realm, revealing that what we perceive as "heat" is actually the frantic dance of atoms and molecules.
In temperature, everything starts from absolute zero:
The concept of absolute zero (-273.15°C or 0 K) is not merely a mathematical convention, but a physical limit imposed by the laws of thermodynamics. The third law formally states:

Where S represents the entropy of the system and S₀ its value in the ground state, in practice, this principle implies that reaching absolute zero is impossible, although modern laser cooling techniques have achieved nanokelvin temperatures, approaching this cosmic limit. The path to precise temperature measurement began with qualitative devices such as Galileo's thermoscope (1592), which lacked a numerical scale. The major innovation came with liquid-in-glass thermometers, whose operation is based on the thermal expansion equation
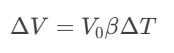
Where:
-
ΔV = volume change
-
V₀ = initial volume
-
β = coefficient of thermal expansion
-
ΔT = temperature change
Later, more robust and specialized sensors were developed for each specific situation, for example:
Thermocouples: These work through the Seebeck effect, which is the union of two different metals. The most common are type K (chromel-alumel) and type J (iron-constantan). When subjected to temperature changes, they generate an electrical potential difference. These sensors are very robust, have a long measuring range, are inexpensive, and have a very fast response time. However, their disadvantage is that this potential difference is not linear, and their accuracy is not very good.
RTDs: They work through a thermoresistive effect, it is an electrical resistance that increases its value in ohms (Ω) as its temperature increases. They exist in various materials, the most common is platinum (PT100), which indicates that it has a resistance of 100 Ω at 0 ° C, but it can also be PT1000, which has 1000 Ω at 0 ° C. This value can be measured and is known as R₀. They exist in different classes and different sensitivities. They can be very precise if they are calibrated through characterization by the Callendar Van Dusen method or the ITS-90 method, in which the behavior of the resistance vs. temperature is obtained through coefficients that are entered into some industrial and scientific readers. Their disadvantages are that they have limited ranges at high temperatures, normally and commercially they only measure up to 660 ° C, they are slow to take the measurement (stabilize), and they are very prone to corrosion. They can crack and stop measuring due to thermal stress (going from low to high temperatures in a short period of time).
Even in recent years, infrared thermography (pyrometers and thermal cameras) has gained significant importance due to its ability to measure in hostile or high-temperature environments, thus avoiding contact temperature measurements and preventing damage or accidents. This technology measures the temperature of an object by detecting the infrared radiation it emits, based on the Stefan-Boltzmann law. This technology has advanced significantly since the invention of the bolometer around 1880 by Samuel Pierpont Langley, and with thermal cameras, predictive maintenance can be performed and failures can be anticipated.
In measuring thermal thermography, two terms normally arise: heat and temperature, which we explain:
Heat is a form of energy in transit that flows spontaneously from warmer to colder bodies. Its study revolutionized science in the 19th century with the development of thermodynamics:
-
Molecular kinetic energy: Heat manifests as the disordered motion of atoms and molecules (kinetic theory)
-
First Law of Thermodynamics: ΔU = Q - W, where heat (Q) contributes to the internal energy (U) and W represents the thermodynamic work performed by the system on its environment.
-
Transmission methods: Conduction (solids), convection (fluids), and radiation (vacuum)
Temperature is a measure of the average kinetic energy of particles. Understanding it took centuries of development:
• Historical scales: From the arbitrary degrees of Fahrenheit (1724) to the absolute Kelvin scale (1854)
• Absolute zero (-273.15°C): Thermodynamic limit where molecular motion ceases
• Instrumentation: Evolution from Galileo's thermoscopes to modern optical pyrometers.
To measure temperature, we use different reference scales, each with its own characteristics and applications. For example:
Celsius (°C)
Definition: Based on the melting (0°C) and boiling (100°C) points of water at 1 atm.
Usage: Metric system, science, and everyday life in most of the world. Incidentally, in some places, the scale is still called degrees Celsius. However, metrologically, this term has been obsolete since 1948, when the General Conference on Weights and Measures (CGPM) changed it to avoid confusion with the term angular unit (1/100 of a degree).
Conversion formula:

Kelvin (K)
-
Definition: Absolute scale where 0 K = -273.15°C (absolute zero, absence of molecular motion).
-
Use: Thermodynamics, quantum physics, and astronomy.
-
Conversion formula:

Fahrenheit (°F)
-
Definition: Originally based on a mixture of ice, water, and sal ammoniac (0°F) and human body temperature (~96°F, later adjusted to 98.6°F).
-
Use: Primarily in the U.S. and some English-speaking countries.
-
Conversion formula:

Rankine (°R)
-
Definition: Absolute scale based on Fahrenheit (0°R = -459.67°F = absolute zero).
-
Use: Thermal engineering in English systems.
-
Conversion formula:

.png)